If you are a candidate for the CoolSculpting® Elite treatment, your doctor will work with you to create a customized treatment plan depending on your goals.
Here’s an idea of what you can expect during treatment.
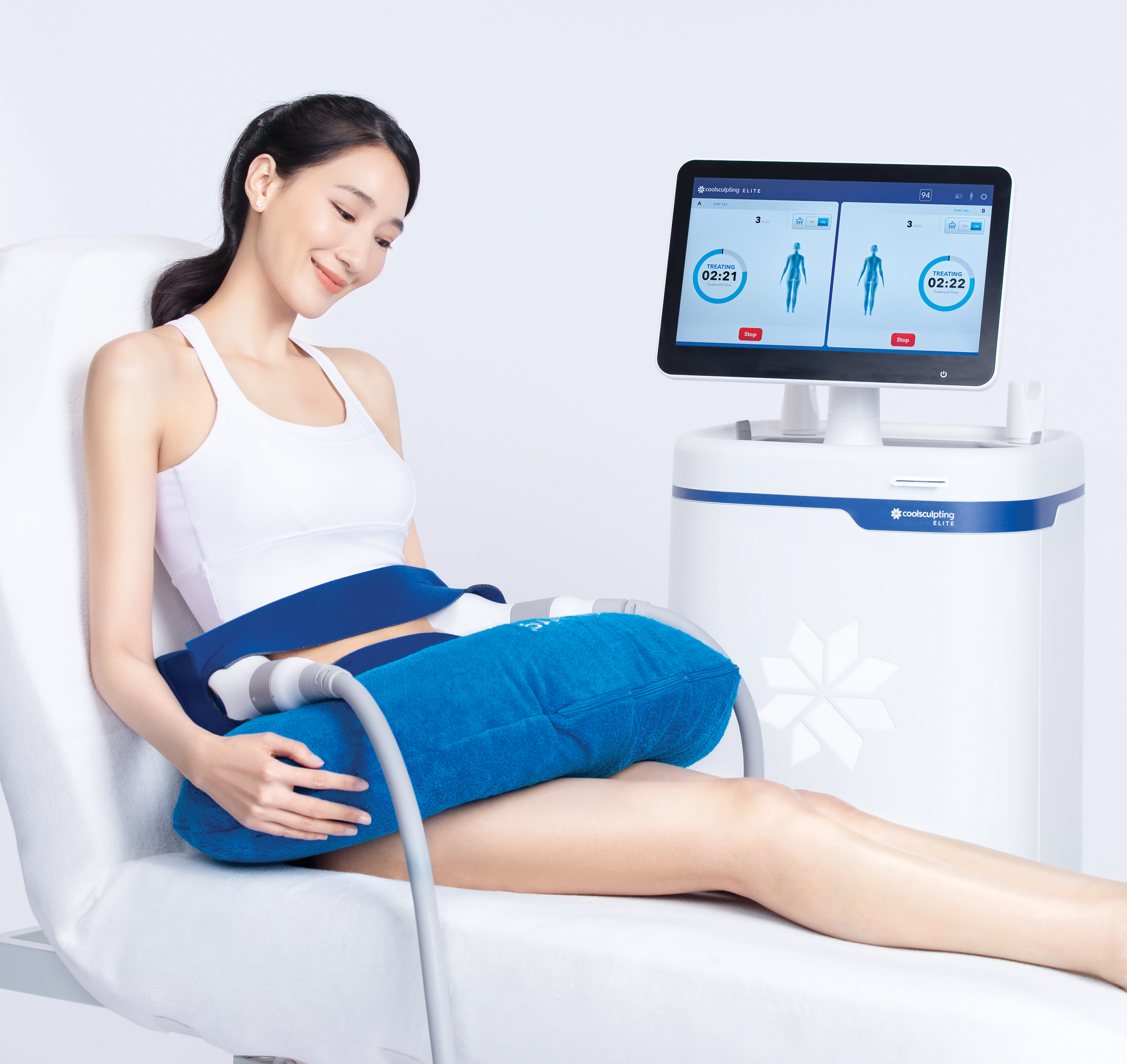

Get prepped
Once you and your doctor have decided on your treatment areas, your provider will apply a clear gel pad to create a barrier between the cooling panel and your skin. Clinical trials have demonstrated CoolSculpting® to be safe and effective in reducing fat in 9 areas of the body. CoolSculpting® Elite's dual applicators allow you to treat 2 areas at once.


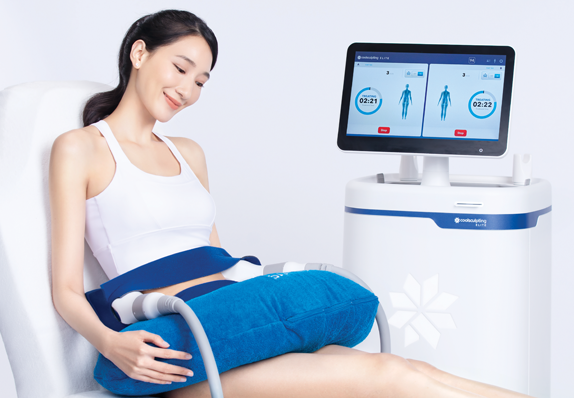
Let science go to work
You'll feel a slight sucking sensation as the applicator adheres to your body. During the procedure you may experience sensations of pulling, tugging, mild pinching and cold at the treatment site. These sensations subside as the area becomes numb.



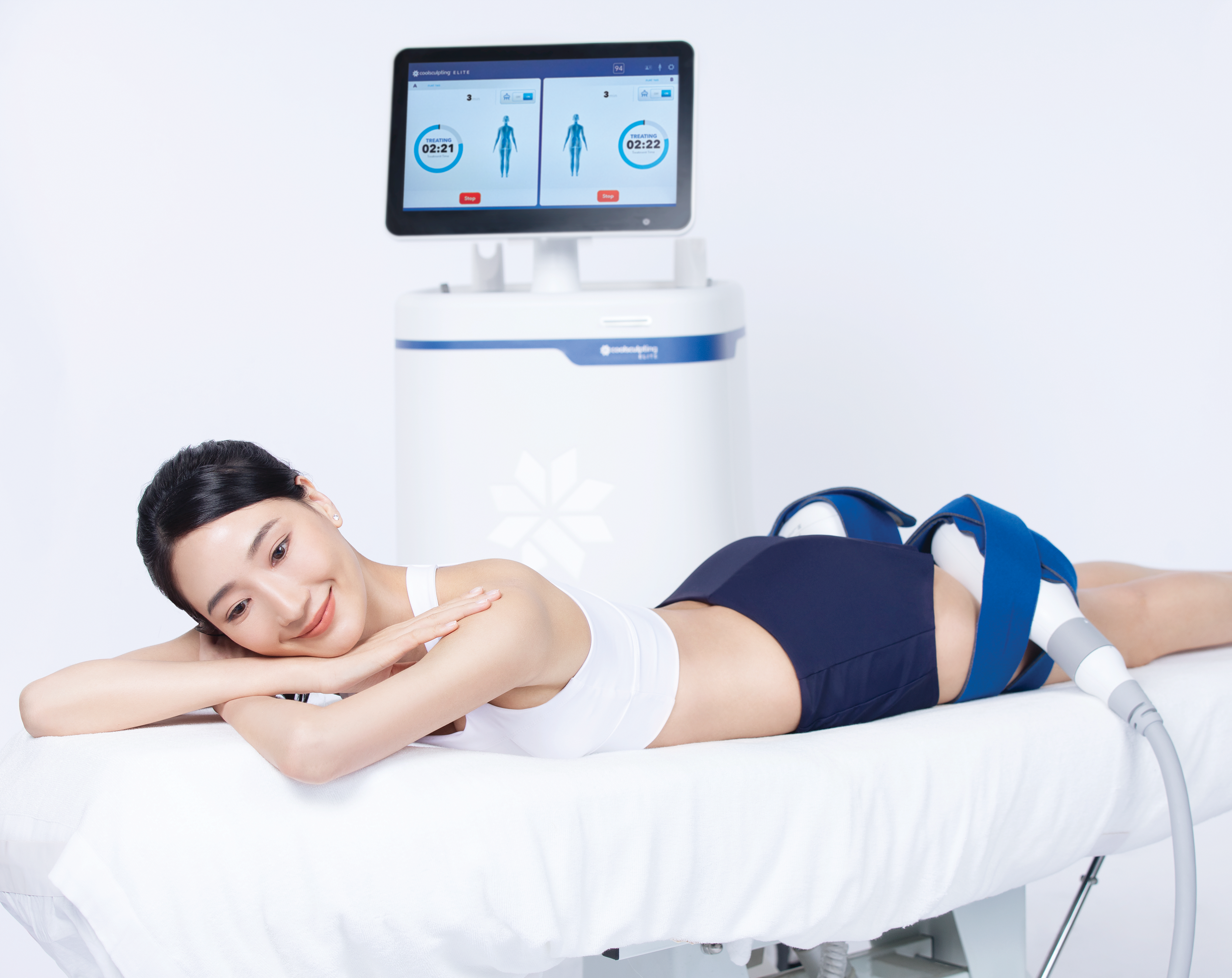
And Chill
During the treatment you'll be able to read, answer emails, or chat with a friend. After the applicator is detached, your provider will do a brief massage on the area(s) to break up the treated fat cells and enhance the fat reduction. That's it! Most patients experience little to no downtime after treatment.

One treatment with CoolSculpting® Elite reduces up to 27% of fat cells in the treated area6
You may need more than one treatment to reach your desired results and get the most out of your CoolSculpting® Elite experience.
Uses:
CoolSculpting® and CoolSculpting®
Elite are FDA-cleared for the
treatment of visible fat bulges in the submental (under
the chin) and submandibular (under the jawline) areas, thigh, abdomen, and
flank, along with bra fat, back fat,
underneath the buttocks (also known as banana roll), and upper arm. It is also
FDA-cleared to affect the appearance of
lax tissue with submental area treatments.
CoolSculpting® and
CoolSculpting® Elite are not treatments for weight
loss.
Important safety information:
During the procedure patients may experience sensations of pulling, tugging, mild pinching, intense cold, tingling, stinging, aching and cramping at the treatment site.13 These sensations subside as the area becomes numb.13
Following the procedure, typical side effects include redness, swelling, blanching, bruising, firmness, tingling, stinging, tenderness, cramping, aching, itching, skin sensitivity and numbness. Numbness can persist for up to several weeks. A sensation of fullness in the back of the throat may occur after submental treatment. The following rare and very rare side effects have the following incidence rates (approximate occurrences per number of treatments): paradoxical hyperplasia (1/3000 [0.033%]); late-onset pain (1/6000 [0.017%]); severe pain (1/6000 [0.017%]); hyperpigmentation (1/11000 [0.009%]); freeze burn (1/15000 [0.006%]); treatment area demarcation (1/20000 [0.005%]); vasovagal symptoms (1/30000 [0.003%]); subcutaneous induration (1/30000 [0.003%]); cold panniculitis (1/60000 [0.002%]) and hernia (1/185000 [0.001%]).13,15 The CoolSculpting® procedure is not for everyone. Patients should not have the CoolSculpting® procedure if they suffer from cryoglobulinaemia, cold agglutinin disease or paroxysmal cold haemoglobinuria.13 The CoolSculpting® procedure is not a treatment for obesity.14 CoolSculpting® is not a weight loss procedure and should not replace a healthy diet and active lifestyle.
Please see CoolSculpting® and CoolSculpting Elite® Important Safety Information for additional information.
Effect of treatment varies with individuals
References:
- Medical Insight Inc. The Global Aesthetic Market Study: Version XVII. 2019
- All time International Cycles Summary_040621. Data sourced from Connect Cube database [provided by International Business Excellence]. May 2021.
- Manstein D et al. Lasers Surg Med 2008; 40: 595-604
- Zelickson B, et al. Derm Surg 2009; 35:1462-70
- Allergan Inc. Unpublished Data. CoolSculpting® Publications. INT-CSC-2050471. December 2020.
- Sasaki GH, et al. Aesthet Surg J 2014; 34:420–31
- Allergan. Unpublished data. INT-CSC-2050028. CoolSculpting® clinical “Fit and Function” study to test the V003 wells. February 2020.
- Allergan. Unpublished data. INT-CSC-2050288. Applicator cooling and distribution dye test. August 2020.EN-A.
- Allergan. CoolSculpting® system (CoolSculpting® ELITE) user manual. CS-UM-CM3-04-EN-A. 2020.
- Manstein D et al. Lasers Surg Med 2008; 40: 595-604
- Avram MM and Harry RS. Lasers Surg Med 2009; 41:703-8
- Krueger N, et al. Clin Cosmet Investig Derm 2014; 7:201-25
- Allergan, CoolSculpting® System User Manual. BRZ-101-TUM-EN6-K
- FDA 510(K) Summary Document. Available at: https://www.accessdata.fda.gov/cdrh_docs/pdf19/K193566.pdf [Accessed November 2021].
- Allergan Inc. US CoolSculpting® Important Safety Information. November 2021. Available at: https://www.coolsculpting.com/pdfs/CSC125713-v3-CoolSculpting-Important- Safety-Information.pdf [Accessed November 2021]

 Find a Clinic
Find a Clinic

